The 2023 theme for World Malaria day is, “Time to Deliver Zero Malaria: Invest, Innovate, Implement.”
The Eck Institute for Global Health is committed to continuing the University of Notre Dame’s history of excellence in vector biology research through its efforts to eliminate malaria worldwide. Through interdisciplinary collaborations, faculty and students from across the University’s campus and around the world are contributing their research expertise to discover solutions that minimize the effects of malaria and other vector-borne diseases, especially in resource-poor countries.
Highlighting EIGH’s Current Malaria Researchers
Invest: Expansion of vector-borne research expertise

“If approximately eight Notre Dame Stadiums were filled to capacity, you would amass a crowd of 619,000 people. The size of this gathering represents how many people died of malaria in 2021,” reflects Lee Haines, research associate professor in the Department of Biological Sciences. Haines joined The University of Notre Dame and the Eck Institute for Global Health in 2023 from Liverpool School of Tropical Medicine.
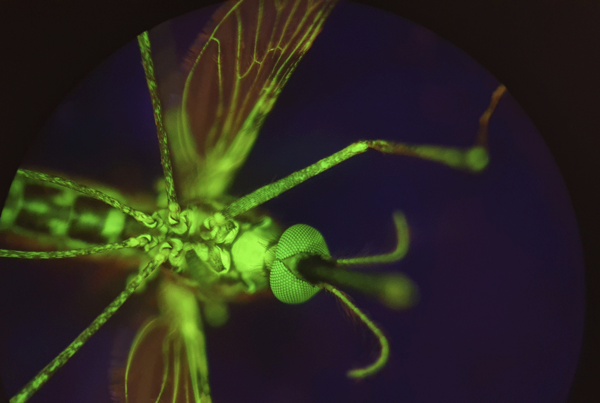
Haines’s research focuses on diseases that are spread by arthropods, particularly the interrelationships between insects (tsetse, sand flies and mosquitoes), their microbiota, and the pathogens they transmit. Actively engaged in research in the areas of molecular biology and parasitology, Haines, while at the Liverpool School of Tropical Medicine, has been working on a project aiming to establish a link between the microbiomes of mosquitoes and insecticide resistance. A passionate science ambassador and public speaker, she has also captured award-winning images of arthropod vectors, including Anopheles gambiae, the most lethal vector of human malaria.

Previously at the Liverpool School of Tropical Medicine, Álvaro Acosta-Serrano has joined the Eck Institute for Global Health as a professor in the Department of Biological Sciences. An expert in vector biology and parasitology, Acosta-Serrano was the recipient of the 2022 Wright Medal of the British Society for Parasitology. His research focuses on “fundamental aspects of the biology of kinetoplastid parasites and their vectors, and on developing tools to control the transmission of vector-borne neglected diseases and malaria." A prolific writer and researcher, he has served as an academic editor for several journals on several scientific committees and networks. Research in the Notre Dame Acosta-Serrano lab targets the development of molecular tools to control and prevent parasite transmission in disease-endemic areas.
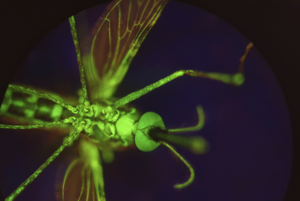
Over the past 5 years, Acosta-Serrano and Haines have collaborated on investigations targeting the control of Anopheles gambiae. Funded by the Bill & Melinda Gates Foundation, and the RoseTreesTrust, their work has focused on the mosquitocidal properties of the 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitors, which are traditionally used for herbicidal application to prevent plant growth. One specific repurposed herbicide, known as the human drug nitisinone, blocks the HPPD enzyme in the tyrosine metabolism pathway that, when inhibited, kills mosquitoes and other blood-feeding insects. Insects that do not feed on blood (such as pollinator species) are not affected, making HPPD inhibitors a more environmentally-friendly alternative to current neurotoxic insecticides. Current projects at the University of Notre Dame are expanding upon the tests with nitisinone as an ectoparasitic drug administered to humans or animals in hopes of rendering their blood mosquitocidal.
Innovate: Mapping determinants of drug resistance in malaria parasites
Supported as an Eck Institute for Global Health 2022-2023 Graduate Research Fellow, Mac Sievert is a member of the Ferdig Lab, led by principal investigator, Michael Ferdig. The Ferdig lab continues to develop the tools and computational framework to reveal and collect the genetic architecture of complex traits. Applying new technologies and analytical innovations to genetic cross tools such as quantitative trait locus (QTL) mapping, research is directed towards the discovery of key gene interactions which may play a part in drug resistance traits of the most lethal malaria parasite species, Plasmodium falciparum.
Anti-malarial treatments have traditionally included chloroquine and more recently artemisinin-based combination therapies (ACTs). Although chloroquine was successful in treating historical cases of malaria, drug resistance has become fairly common, rendering it an ineffective treatment option in many parts of the world. Artemisinin-resistant genes have also emerged, particularly in Southeast Asian countries. At the Ferdig Lab, a custom high-density microarray assayed genome-wide copy number variations (CNVs) and 45,524 single nucleotide polymorphism (SNP) loci of 160 Plasmodium falciparum parasites from Thailand, Laos, Cambodia, Malawi, and the Gambia to identify evidence of selection and their potential phenotypic impact.
Researchers are currently utilizing QTL mapping to connect genes of Plasmodium falciparum with the potential for drug-resistance phenotypes. The genetic crosses which were created through the use of a novel humanized-liver mice model, have produced over 600 progeny for study. In partnership with the National Institutes of Health (NIH), the National Center for Advancing Translational Sciences (NCATS) was utilized to test the progeny samples against thousands of drugs, and to deliver results back to the Ferdig Lab. A statistical analysis and evaluation of the data is being conducted by Sievert and others which will be delivered to NIH to provide promising advancements in developing effective ACTs.
“The application of our genetic crosses with NCATS’ drug screening allows us to make the most of our innovative methods for studying drug susceptibilities and resistances,” said Sievert. “By determining how resistance happens and how it spreads, we can identify the most successful drug combination therapies,” an important step towards reducing and eliminating malaria.
Implement: AEGIS Project: Trials commence in Kenya

Notre Dame researchers including John Grieco, Nicole Achee, Fang Liu, Alex Perkins, and Sean Moore of the Unitaid-funded project AEGIS (Advancing Evidence for the Global Implementation of Spatial Repellents) are conducting fieldwork in Kenya in collaboration with Kenya Medical Research Institute (KEMRI) and the CDC to evaluate the effects of spatial repellents (SRs) as a cost-effective control tool for combating malaria. Spatial repellents release chemicals into the air to prevent mosquitoes from biting humans within a given space, thus helping to prevent disease transmission.
Despite prevention efforts such as nets and sprays, mosquitoes are still finding ways to bite and infect humans. “Mosquitoes are smart, and they are biting earlier and later in the day and are changing their behaviors faster than we are,” explains Grieco. When used in combination with long-lasting insecticide nets (LLINs) and indoor residual spraying (IRS), SRs are a way to deliver dramatic reductions in malaria transmission in communities where millions of people suffer and die every year from mosquito-borne diseases.
At the beginning of the Kenya trial, study members placed hooks on the walls where the SRs were then installed. Each month, the team members have returned to replace the SRs with a new product. Efficacy trials will also be conducted in Sri Lanka and Mali, with an independent Uganda operational study to follow, which will help to generate data on the effectiveness of SR product delivery mechanisms. AEGIS will provide the World Health Organization with the data it needs to make an informed decision on making SRs available to those who need them most.
Research in support of the Ministry of Health and Wellness in Belize’s efforts to achieve certification of malaria elimination is also ongoing at the Belize Vector and Ecology Center, which is co-directed by John Grieco and Nicole Achee. John and Nicole also support efforts in the Ivory Coast to evaluate the efficacy of eave tubes which utilize special insecticide-laced netting to kill mosquitoes as they attempt to enter the eaves of traditional African homes.
Contact:
Christine Grashorn, Communications Specialist
Notre Dame Research / University of Notre Dame
cgrashor@nd.edu / 574.631.4856
research.nd.edu / @UNDResearch
About the Eck Institute for Global Health
The Eck Institute for Global Health (EIGH), an integral part of Notre Dame Research, builds on the University’s historical strength in infectious disease research while broadening the scope of our expertise to include Epidemiology, Molecular biology and microbiology, Computational science, Community health, Genetics and genomics, Biochemistry, Non-communicable diseases and Social sciences. Our team of interdisciplinary researchers and their students holistically address health disparities around the world. EIGH faculty affiliates recognize health as a fundamental human right and promote research, training, and service to advance health standards for all people, especially those in resource-poor countries who are disproportionately impacted by preventable diseases. The EIGH is training the next generation of global health researchers and leaders through undergraduate, Master of Science in Global Health, doctoral, and postdoctoral programs.
Originally published by at globalhealth.nd.edu on April 25, 2023.
